Abstract
BACKGROUND: Acute Graft versus Host Disease (aGVHD) represents a serious and sometimes life-threatening condition associated with patients undergoing allogeneic hematopoietic stem cell transplantation (HSCT). Development of aGVHD is characterized by increased levels of inflammatory mediators and activated T cells in circulation, leading to tissue and organ damage. Corticosteroids are primarily utilized as first line treatment for aGVHD; however, this approach does not provide therapeutic benefit for a significant portion of aGVHD subjects. The combination of itacitinib, a JAK1-selective inhibitor, with corticosteroids was evaluated in a parallel-cohort phase 1 trial (NCT02614612) and resulted in improved overall responses for both steroid naïve and refractory aGVHD subjects.
Previous studies have demonstrated that a panel measuring the plasma levels of ST2, REG3A, and TNFR1 can be utilized to distinguish steroid treated subjects with an increased risk of progressive aGVHD; however, it is unknown if these biomarkers are predictive of response to the combination of corticosteroids and itacitinib. In this study, we compared the plasma levels of ST2, REG3A, TNFR1, and Trappin-2/Elafin in order to distinguish response to combination treatment. Additionally, we conducted a broad proteomic analysis to identify potential prognostic biomarkers of response to the combination treatment.
METHODS: Plasma samples were collected from 31 subjects enrolled in the Phase 1 clinical trial prior to and at designated times following treatment. Levels of REG3A, ST2, TNFR1, and Trappin-2/Elafin were measured using SimplePlex multiplex platform based on manufacturer instructions. Broad proteomic analysis was additionally conducted by OLINK Proteomics using a proximity extension assay. Statistical differences were identified using unpaired T tests and significance conferred when p<0.05. All subjects provided written consent prior to enrollment.
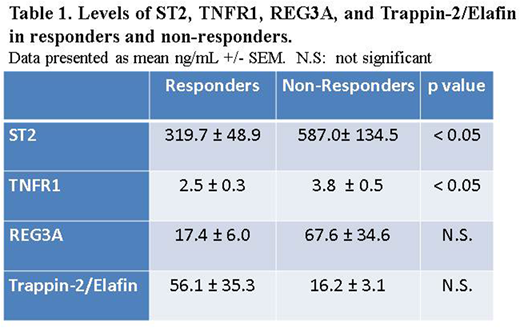
RESULTS: Subjects were separated into responders (10- Complete Responder, 1-Very Good Partial Responder, 8-Partial Responder) and non-responders (2-Mixed Responder, 10-Progressive Disease/Death) based on CIBMTR response criteria at day 28. Levels of ST2 and TNFR1 were significantly elevated at baseline in non-responders (p<0.05 for both ST2 and TNFR1) when compared with responders (Table 1). Additionally, baseline levels of ST2 and TNFR1 significantly correlated (p<0.0001 for both ST2 and TNFR1) with the Minnesota and CIBMTR response criteria for aGVHD. There were no significant differences in the levels of REG3A and Trappin-2/Elafin between responders and non-responders.
Novel biomarkers were identified and associated with therapeutic response by comparing the levels of approximately 1000 proteins in the CR and PD/Death cohorts specifically. The initial analysis identified 44 proteins significantly upregulated (p<0.05; fold change > 1.5) and 61 proteins significantly down-regulated (p<0.05; fold change < -1.5) in the CR cohort at baseline compared with the PD/Death cohort. Further analysis of the CR cohort identified 23 proteins significantly modulated (Fold Change > |1.5|, p<0.05) between baseline and day 28 samples which may serve as additional biomarkers of therapeutic response.
CONCLUSION: Proteomic analysis of clinical samples confirmed the value of ST2 and TNFR1 in predicting response to treatment with itacitinib and steroids and also identified a number of novel plasma biomarkers that may have utility in selecting and/or monitoring aGVHD subjects when treated with a combination of corticosteroids and itacitinib.
Pratta:Incyte Research Institute: Employment, Equity Ownership. Liu:Incyte Research Institute: Employment, Equity Ownership. Arbushites:Incyte Corporation: Employment, Equity Ownership. Newton:Incyte Research Institute: Employment, Equity Ownership. Howell:Incyte Research Institute: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal